
ACCELERATING
Clinical Rx Development
and Diagnostic Research toward Commercialization

DIAGNOSTICS PORTFOLIO
(INCELLKINE ASSAY)
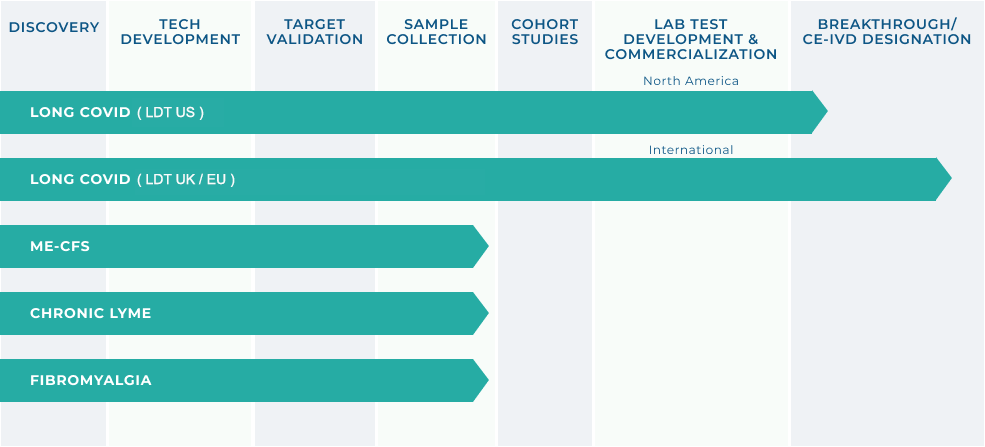
- Lab partners located in USA and Globally to process patient testing

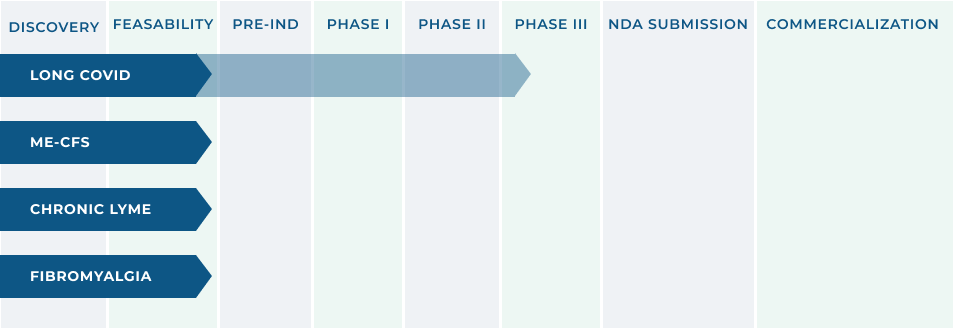
RX THERAPEUTIC INNOVATION
In January, 2024, our application was accepted by the FDA to proceed with a Randomized Clinical Trial for the Treatment of Long COVID/Post Acute Sequelae of COVID (PASC) with Selzentry® (maraviroc) in Combination with Lipitor (atorvastatin). The clinical trial will support a New Drug Application (NDA) filing through the 505(B)(2) pathway of the Federal Food, Drug, and Cosmetic Act (FDCA).
The initial focus is on the diagnosis and treatment of Long Covid with ongoing evaluation of other indications that present significant unmet medical needs.

BIOMETRIC REMOTE MONITORING


An integral personal device for your immune health and wellness
Long-lasting battery
7-10 day long-lasting battery life
water & shock resistant
5 ATM Water and Shock Resistant
VITAL HEALTH TRACKING
Tracks multiple vital health functions and activities important to managing your immunologic symptoms
30 DAY DATA PERIOD
A month of health data memory
continuous tracking
Allows uninterrupted tracking for longer intervals for more accurate tracking
FCC & IC COMPLIANT
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to Part 15 of the FCC Rules
